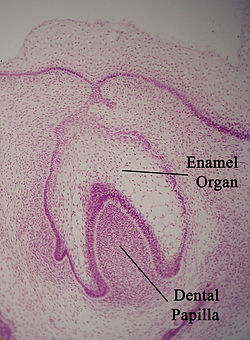
Enamel organ
| Enamel organ | |
|---|---|
 Enamel organ | |
| Details | |
| Identifiers | |
| Latin | organum enameleum |
| MeSH | D004658 |
| TE | organ_by_E5.4.1.1.2.3.5 E5.4.1.1.2.3.5 |
| Anatomical terminology | |
The enamel organ, also known as the dental organ, is a cellular aggregation seen in a developing tooth and it lies above the dental papilla.[1] The enamel organ which is differentiated from the primitive oral epithelium lining the stomodeum. The enamel organ is responsible for the formation of enamel, initiation of dentine formation, establishment of the shape of a tooth's crown, and establishment of the dentoenamel junction.[1]
The enamel organ has four layers; the inner enamel epithelium, outer enamel epithelium, stratum intermedium, and the stellate reticulum.[1]
The dental papilla, the differentiated ectomesenchyme deep to the enamel organ, will produce dentin and the dental pulp. The surrounding ectomesenchyme tissue, the dental follicle, is the primitive cementum, periodontal ligament and alveolar bone beneath the tooth root.[1] The site where the internal enamel epithelium and external enamel epithelium coalesce is the cervical root, important in proliferation of the dental root.[1]
Tooth development
[edit]Tooth development begins at week 6 in utero, in the oral epithelium. The process is divided into three stages:
- Initiation
- Morphogenesis and
- Histogenesis[2]
At the end of week 7 i.u., localised proliferations of cells in the dental laminae form round and oval swellings known as tooth buds, which will eventually develop into mesenchymal cells and surround the enamel organ. Each epithelial swelling and the surrounding mesenchymal cells form a tooth germ.[3]
Tooth germs are the primitive structure of teeth; their formation is in three distinct stages: bud stage, cap stage, bell stage.
The stages are based on the degree of development of enamel organ. Oral epithelium forms the tooth enamel while the ectomesenchyme forms the pulp and dentine of the tooth. The ectomesenchyme lies deep to the oral epithelium.[4]
Bud Stage
[edit]This is the initial stage of tooth development, which occurs at week 8 i.u.. Proliferation of dental lamina occurs, forming small tooth buds which are spherical or ovoid condensations of epithelial cells, now known as the enamel organ.[1] The enamel organ consists of peripherally located, low columnar cells and centrally located polygonal cells. The enamel organ is also surrounded by proliferating mesenchymal cells, which results in the condensation of two distinct areas:[2]
- The dental papilla: below the enamel organ
- The tooth sac: ectomesenchymal condensation of the area surrounding the tooth bud and dental papilla.
Both the dental papilla and the tooth sac are not structurally defined in the bud stage, and will become more defined in subsequent stages (Cap and Bell stages). The interaction and signalling between the enamel organ and the surrounding mesenchymal cells play an important role in the later stages of tooth development.[2] Each dental arch will have 10 tooth buds, accounting for 20 primary teeth.
Cap Stage
[edit]The cap stage occurs in week 9-10 i.u.[1] Unequal proliferation of cells during this stage, invaginating into the ectomesenchyme tissue, leads to the formation of the cap-shaped enamel organ. The ectomesenchyme tissue also invaginates superficially to shape the primitive dental pulp. Differentiation of cells occurs at this stage to make different tissue layers; external enamel epithelium, stratum intermedium, stellate reticulum, internal enamel epithelium, dental papilla, and dental follicle. The external enamel epithelium, a layer of simple cuboidal epithelium, has a protective role during tooth development.[1] The stellate reticulum, the innermost layer of the enamel organ, gathers GAGs between cells. The internal enamel epithelium will form enamel during the Bell Stage
Early Bell stage
[edit]There is uneven growth of enamel organ in this phase, and the epithelial cap deepens.[3] The cap shape of the enamel organ assumes a bell shape as the undersurface of the cap deepens.[3] Foldings of the internal enamel epithelium (done by the growing papilla cells) maps out the occlusal pattern of the tooth crown. The process is known as morphodifferentiation. The pressure exerted by the dental papilla cells has been shown to be opposed equally by the pressure from the fluid in the stellate reticulum (present in the enamel organ).[3]
The folding of the enamel organ is caused by different rates of mitosis and difference in cell differentiation times, causing different crown shapes in each tooth.
Late Bell stage
[edit]This stage is the apposition stage (formation of dental hard tissues), also characterised by the commencement of root formation and mineralisation. The area between the internal enamel epithelium and odontoblasts outline the future dentinoenamel junction. Formation of dentine (dentinogenesis) precedes enamel formation (amelogenesis). It occurs first as along the future dentinoenamel junction in the region of future cusps and proceeds pulpally and apically. Cells of the internal enamel epithelium become pre-ameloblasts and release inductive factors which encourage the differentiation of odontoblasts from the mesenchymal cells of the dental papilla.[1] This can be seen in the figure (marked A). The odontoblasts lay down dentine (see pale blue band). After the first layer of dentine is formed, this induces ameloblasts (B) to lay down enamel (red region) over the dentine in the future incisal and cuspal areas. Amelogenesis will then follow. The cervical portion of the enamel organ then gives rise to the Hertwig Epithelial Root Sheath (HERS)- this outlines the future root and also is responsible for the size, shape, length and the number of roots.
Determination of crown morphology
[edit]The composition of the enamel organ does not vary greatly between incisors, canines, premolars, and molars. Although the quantity of odontoblasts, ameloblasts and cementoblasts present in premolars/molars and incisors/canines remains the same, the major difference between these morphological types of teeth is the rate of secretion and quantity of products secreted by the enamel organ (dentine, enamel, cementum). There has been no definite consensus as to what determines the differences between enamel organs in different teeth. However, it is a widely held view by dental professionals and biologists that genes [5] and cell signaling[6] between cells in the dental extracellular matrix/enamel matrix play a role.
The shape of the enamel layer covering the crown is determined by five growth parameters:[7]
- The appositional growth rate
- Duration of appositional growth (at the cusp tip)
- Ameloblast extension rate
- Duration of ameloblast extension
- Spreading rate of appositional termination.
The appositional growth mechanism establishes the thickness of the enamel layer and it is determined by ribbon-like carbonate apatite crystals which are present in the rods (or prisms)[1] and interrods. They are produced by the ameloblast in the bell stage of tooth development. As the crystals are long and closely packed, the thickness depends on the abundance of the crystals in the tooth. Crown shape or morphology is determined by the epithelial-mesenchymal interaction, which occurs at the dentinoenamel junction (DEJ). Firstly, the pre-ameloblasts differentiate from the inner enamel epithelia on the dentine surface covering the pulp horn.[8] A wave of ameloblasts will then differentiate from the cusp tip and move through the inner enamel epithelia down the slope of the mineralised dentine surface. The differentiation will extend down the slope of the dentine surface and reaches its limit, where the inner epithelium is fused with the outer enamel epithelium to form Hertwig's epithelial root sheath. Enamel mineral will increase daily (apposition growth) during the secretory stage of amelogenesis (enamel formation). Ultimately, the secretory stage will end and they will transition into maturation stage ameloblasts. These ameloblasts will move down to the enamel surface of the tooth and the shape of the crown and tooth is then established.[9]
Abnormalities
[edit]Odontomes
[edit]Odontomes are considered to be developmental anomalies resulting from the growth of completely differentiated epithelial and mesenchymal cells that give rise to ameloblasts and odontoblasts.[10] Histologically, they are composed of different dental tissues including enamel, dentine, cementum[11] and in some cases, pulp tissue, therefore if the enamel organ is not arranged in its proper fashion, an odontome may form.[12] Odontomes are categorised as either:
- Compound
- this malformation is anatomically like a normal tooth, and has dental tissues (enamel, dentine, cementum) placed in an orderly fashion. These are more frequent than complex odontomes.[12][13]
- Complex
- this malformation results in dental tissues being arranged in a disorderly fashion, forming an irregular mass.[12][13]
Odontomes are rare entities and usually asymptomatic; they are often incidental findings on routine dental radiographic examinations.[14][15] The complex odontome appears as an irregular mass of calcified material surrounded by a thin radiolucent area with smooth periphery, and the compound type shows calcified structures resembling teeth in the centre of a well-defined radiolucent lesion.
Some factors related to the development of odontomes are:
- Changes in genetic components responsible for tooth development
- Trauma at primary dentine period
- Inherited conditions such as Gardner's Syndrome
- Infection
- Inflammation
- Hyperactivity of odontoblasts.[12][15]
The first reported case of an odontome erupting in the oral cavity was in 1980.[15]
Dens Invaginatus
[edit]Dens Invaginatus is a dental anomaly which means that the affected tooth (dilated odontome) contains a cavity that is completely or partially lined by enamel, radiographically resembling a tooth within a tooth (dens in dente).[16]
There is a lack of consensus on the aetiology of dens invaginatus. It is suggested that dens invaginatus arises because during odontogenesis, there is proliferation and ingrowth of the cells of the enamel organ into the dental papilla during development.[17]
Another proposed theory is that the distortion of the enamel organ during tooth development and subsequent protrusion of a part of the enamel organ will lead to the formation of an enamel-lined channel ending at the cingulum or occasionally at the incisal tip.[18]
Histologically, there are differences in the structure and composition between the external and internal enamel in dens invaginatus. The internal enamel exhibits atypical and more complex rod shapes and its surface has the typical honeycomb pattern but no perikymata.[19]
The invagination can be:
- Coronal type: slight pitting involving the enamel organ infolding into the dental papilla
- Radicular type: occupying most of the crown and root involving invagination of Hertwig's epithelial root sheath, lined with cementum.[20]
Dens invaginatus has a clinical importance as teeth affected with dens invaginatus are predisposed to developing pulpal disease. The invagination allows entry of irritants into an area which is separated from pulpal tissue by only a thin layer of enamel and dentine and extra preventative measures are advised to prevent dental caries.[21]
Enamel Defect and Coeliac Disease
[edit]Coeliac disease in children is thought to be underdiagnosed because it may initially be asymptomatic. Studies have shown that enamel defect of permanent and deciduous or primary teeth may suggest the presence of undiagnosed coeliac disease in children and adults.[22][23][24] Coeliac disease-related enamel defects are most commonly associated with incisors and first molar teeth, and are characterised by symmetrical distribution of enamel defects on the same tooth in all 4 quadrants.[22][25] This is a distinct characteristic of enamel defects in coeliac disease that cannot be seen in other enamel defects.
Enamel defects in coeliac disease occur due to an interference in tooth formation by amelogenin. Amelogenin is a proline-rich enamel protein that plays a major role in mineralisation and organisation of tooth crystals.[26][27] Disruption to this process cause alterations in the tooth surface. Patients with coeliac disease produce high levels of circulating IgG and IgA antigliadin antibodies (AGA) in order to get rid of protein gliadin, which is toxic to these patients. However, due to the structural similarities between amelogenin and gliadin, AGA may interfere with amelogenin which lead to improper formation of enamel.[26] Furthermore, because IgG can be transported across the placenta, the amelogenesis process is disturbed from the intrauterine period to adolescence.[25]
Gliadins are highly hydrophobic proteins in wheat gluten. The antibodies are produced to interact with this protein. Therefore, a gluten-free diet may lead to normalisation of tooth development as circulating antibodies for enamel defect may decrease.[28]
See also
[edit]References
[edit]- ^ a b c d e f g h i j Antonio N (2017-10-13). Ten Cate's oral histology: development, structure, and function. review (9th ed.). St. Louis, Missouri: Elsevier. ISBN 978-0-323-48524-1. OCLC 990257609.
- ^ a b c Avery J (1951). "Embryology of the tooth". Journal of Dental Research. 30: 490.
- ^ a b c d Avery J (1954). "Primary induction of tooth formation". Journal of Dental Research. 33: 702.
- ^ Pansky B (1982). Review of MEDICAL EMBRYOLOGY. Ohio. p. 77.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Rauth RJ, Potter KS, Ngan AY, Saad DM, Mehr R, Luong VQ, Schuetter VL, Miklus VG, Chang P, Paine ML, Lacruz RS, Snead ML, White SN (December 2009). "Dental enamel: genes define biomechanics". review. Journal of the California Dental Association. 37 (12): 863–8. doi:10.1080/19424396.2009.12223043. PMC 2825347. PMID 20066874.
- ^ Jussila M, Thesleff I (April 2012). "Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages". review. Cold Spring Harbor Perspectives in Biology. 4 (4): a008425. doi:10.1101/cshperspect.a008425. PMC 3312678. PMID 22415375.
- ^ Simmer JP, Papagerakis P, Smith CE, Fisher DC, Rountrey AN, Zheng L, Hu JC (October 2010). "Regulation of dental enamel shape and hardness". review. Journal of Dental Research. 89 (10): 1024–38. doi:10.1177/0022034510375829. PMC 3086535. PMID 20675598.
- ^ Baldock, Richard; Bard, Jonathan; Davidson, Duncan; Morriss-Kay, Gillian (2015-09-23). Kaufman's atlas of mouse development supplement : coronal images. Baldock, Richard,, Bard, Jonathan,, Davidson, Duncan,, Morriss-Kay, Gillian,, Kaufman, Matthew H. Amsterdam. ISBN 9780128009130. OCLC 932060547.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Simmer JP, Papagerakis P, Smith CE, Fisher DC, Rountrey AN, Zheng L, Hu JC (October 2010). "Regulation of dental enamel shape and hardness". Journal of Dental Research. 89 (10): 1024–38. doi:10.1177/0022034510375829. PMC 3086535. PMID 20675598.
- ^ Neville B, Damm DD, Allen C, Bouquot J (2009). Oral and maxillofacial pathology. review (3rd ed.). St. Louis, Mo.: Saunders/Elsevier. ISBN 978-1-4377-2197-3. OCLC 834142726.
- ^ Bhargavan Sarojini S, Khosla E, Varghese T, Johnson Arakkal L (2014). "Eruption of odontomas into the oral cavity: a report of 2 cases". primary. Case Reports in Dentistry. 2014: 639173. doi:10.1155/2014/639173. PMC 4037568. PMID 24900927.
- ^ a b c d Girish G, Bavle RM, Singh MK, Prasad SN (2016-01-01). "Compound composite odontoma". primary. Journal of Oral and Maxillofacial Pathology. 20 (1): 162. doi:10.4103/0973-029X.180982. PMC 4860922. PMID 27194882.
- ^ a b Abdul M, Pragati K, Yusuf C (2014). "Compound composite odontoma and its management". primary. Case Reports in Dentistry. 2014: 107089. doi:10.1155/2014/107089. PMC 4283421. PMID 25587458.
- ^ Vengal M, Arora H, Ghosh S, Pai KM (March 2007). "Large erupting complex odontoma: a case report". primary. Journal of the Canadian Dental Association. 73 (2): 169–73. PMID 17355809.
- ^ a b c Mehta D, Raval N, Udhani S, Parekh V, Modi C (2013). "An unusual case report of erupted odontoma". primary. Case Reports in Dentistry. 2013: 570954. doi:10.1155/2013/570954. PMC 3576803. PMID 23476816.
- ^ Malden N (2013-11-02). "Book review: Oral and Maxillofacial Medicine, the Basis of Diagnosis and Treatment, 3rd edition, by Crispian Scully. Oxford: Churchill Livingstone Elsevier, 2013 (448pp; £49.99p/b). ISBN 978-0-7020-4948-4". Dental Update. 40 (9): 738. doi:10.12968/denu.2013.40.9.738.
- ^ Rushton VE (2006-05-13). "Research summary: Radiographic processing in general dental practice". British Dental Journal. 200 (9): 503. doi:10.1038/sj.bdj.4813528. S2CID 24142205.
- ^ Oehlers FA (November 1957). "Dens invaginatus (dilated composite odontome). I. Variations of the invagination process and associated anterior crown forms". Oral Surgery, Oral Medicine, and Oral Pathology. 10 (11): 1204–18 contd. doi:10.1016/0030-4220(57)90077-4. PMID 13477660.
- ^ Bloch-Zupan A (2014), "Genetic Alterations: Heritable Dentin Defects", The Dental Pulp, Springer Berlin Heidelberg, pp. 155–168, ISBN 9783642551598
- ^ "Radicular dens invaginatus". Dental Abstracts. 53 (2): 77–78. 2008-03-01. doi:10.1016/j.denabs.2007.10.012. ISSN 0011-8486.
- ^ Hülsmann M (March 1997). "Dens invaginatus: aetiology, classification, prevalence, diagnosis, and treatment considerations". International Endodontic Journal. 30 (2): 79–90. doi:10.1111/j.1365-2591.1997.tb00679.x. PMID 10332241.
- ^ a b Salanitri, S.; Seow, W. K. (2013). "Developmental enamel defects in the primary dentition: aetiology and clinical management". Australian Dental Journal. 58 (2): 133–140. doi:10.1111/adj.12039. ISSN 1834-7819. PMID 23713631.
- ^ Calvo, J. C. Llodra; Lozano, J. Maldonado; García, P. Baca; Lafuente, P. Junco; Páez, E. Ortega (2008-07-01). "Prevalence of dental enamel defects in celiac patients with deciduous dentition: a pilot study". Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 106 (1): 74–78. doi:10.1016/j.tripleo.2008.01.022. ISSN 1528-395X. PMID 18585624.
- ^ Cheng, Jianfeng; Malahias, Ted; Brar, Pardeep; Minaya, Maria Teresa; Green, Peter H. R. (2010-03-01). "The Association Between Celiac Disease, Dental Enamel Defects, and Aphthous Ulcers in a United States Cohort". Journal of Clinical Gastroenterology. 44 (3): 191–194. doi:10.1097/MCG.0b013e3181ac9942. ISSN 0192-0790. PMID 19687752. S2CID 9376758.
- ^ a b Sóñora, Cecilia; Arbildi, Paula; Rodríguez‐Camejo, Claudio; Beovide, Verónica; Marco, Alicia; Hernández, Ana (2016). "Enamel organ proteins as targets for antibodies in celiac disease: implications for oral health". European Journal of Oral Sciences. 124 (1): 11–16. doi:10.1111/eos.12241. ISSN 1600-0722. PMID 26712243.
- ^ a b Muñoz, Florencia; Río, Natalia Del; Sóñora, Cecilia; Tiscornia, Inés; Marco, Alicia; Hernández, Ana (2012). "Enamel defects associated with coeliac disease: putative role of antibodies against gliadin in pathogenesis". European Journal of Oral Sciences. 120 (2): 104–112. doi:10.1111/j.1600-0722.2012.00949.x. ISSN 1600-0722. PMID 22409216.
- ^ Moradian-Oldak, Janet (2001-09-01). "Amelogenins: assembly, processing and control of crystal morphology". Matrix Biology. 20 (5–6): 293–305. doi:10.1016/S0945-053X(01)00154-8. ISSN 0945-053X. PMID 11566263.
- ^ Dahlbom, Ingrid; Korponay-szabó, Ilma R.; Kovács, Judit B.; Szalai, Zsuzsanna; Mäki, Markku; Hansson, Tony (2010-02-01). "Prediction of Clinical and Mucosal Severity of Coeliac Disease and Dermatitis Herpetiformis by Quantification of Iga/igg Serum Antibodies to Tissue Transglutaminase". Journal of Pediatric Gastroenterology and Nutrition. 50 (2): 140–146. doi:10.1097/MPG.0b013e3181a81384. ISSN 0277-2116. PMID 19841593. S2CID 43720349.
