Tropane
Appearance
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
N-Methyl-8-azabicyclo[3.2.1]octane
| |||
| Other names
2,3-Dihydro-8-methylnortropidine
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 6379695 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.156.627 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H15N | |||
| Molar mass | 125.211 g/mol | ||
| Density | 0.9259 at 15 °C | ||
| Boiling point | 163 to 169 °C (325 to 336 °F; 436 to 442 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
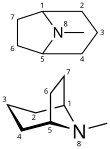
Tropane is a nitrogenous bicyclic organic compound. It is mainly known for the other alkaloids derived from it, which include atropine and cocaine, among others. Tropane alkaloids occur in plants of the families Erythroxylaceae (including coca) and Solanaceae (including mandrake, henbane, deadly nightshade, datura, potato, tomato).[2][3]
Structurally, tropane is cycloheptane with a nitrogen bridge between carbons 1 and 5 and an additional methyl group attached to the nitrogen. While carbons 1 and 5 are asymmetric carbons, tropane itself is optically inactive due to mirror symmetry.
8-Azabicyclo[3.2.1]octane (tropane without the N-methyl group) is known as nortropane or nor-tropane.
See also
[edit]References
[edit]- ^ Merck Index, 11th Edition, 9689.
- ^ "Atropine content of plants". USDA, ARS, National Genetic Resources Program. Phytochemical and Ethnobotanical Databases. [Online Database] National Germplasm Resources Laboratory, Beltsville, Maryland. Archived from the original on November 7, 2004. Retrieved July 25, 2005.
- ^ "Cocaine content of plants". USDA, ARS, National Genetic Resources Program. Phytochemical and Ethnobotanical Databases. [Online Database] National Germplasm Resources Laboratory, Beltsville, Maryland. Archived from the original on November 7, 2004. Retrieved July 25, 2005.